The efficacy of new drugs for multiple myeloma is remarkable! Plan to submit NDA at the end of the year
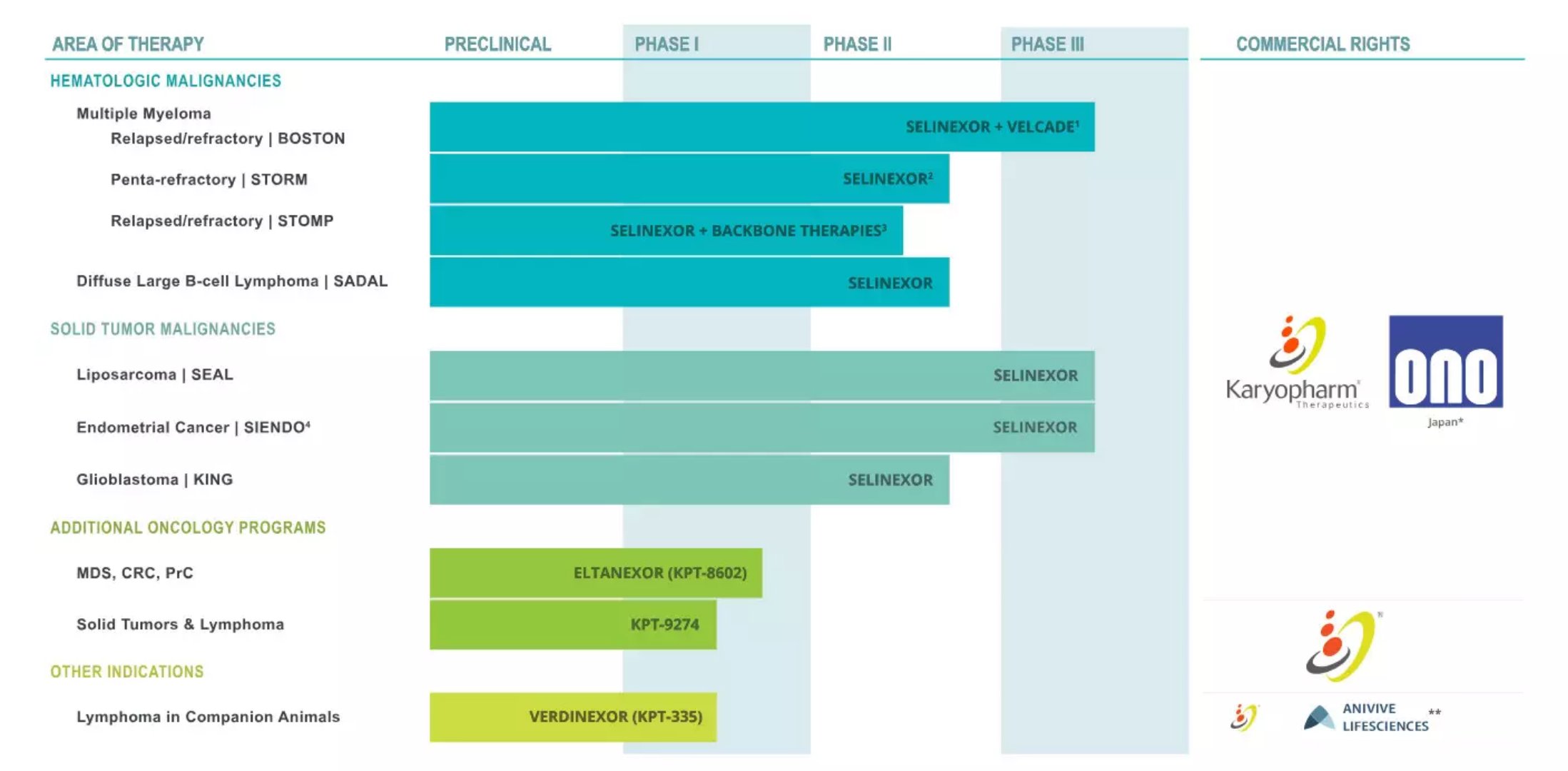
The efficacy of new drugs for multiple myeloma is remarkable! Plan to submit NDA at the end of the year May 02, 2018 Source: WuXi PharmaTech Recently, Karyopharm Therapeutics announced the results of its clinical phase 2b trial of the innovative drug selinexor for the treatment of refractory multiple myeloma (MM). This result indicates that selinexor can produce significant efficacy in highly refractory MM patients who are resistant to five existing therapies. MM is a blood tumor caused by carcinogenesis of plasma cells in the bone marrow. The cancerous plasma cells proliferate in the bone marrow, which not only affects the formation of normal red blood cells and white blood cells, but also causes abnormal accumulation of abnormal antibodies in the body. Patients often experience symptoms such as bone pain, nausea, loss of appetite, frequent infections, and anemia. Although there are many methods for controlling MM, such as chemotherapy, glucocorticoids, and targeted drugs, there are still many patients whose tumors are resistant to all currently approved therapies. These patients urgently need to use innovative mechanisms to control the treatment of MM. The selinexor developed by Karyopharm is an oral selective nuclear export inhibitor that binds to the nuclear export protein XPO1 and inhibits its function, which leads to the accumulation of tumor suppressor proteins in the nucleus. The accumulation of tumor suppressor proteins will reactivate or enhance their tumor suppressor function, and will not have a serious effect on normal cells while inducing specific apoptosis of tumor cells. Selinexor is currently eligible for FDA-approved orphan drug eligibility and fast track. In this multicenter, one-arm, international clinical phase 2b trial called STORM, 122 refractory MM patients who received multiple treatments and developed resistance to 5 different therapies received selinexor with low-dose tamponade Combination therapy consisting of dexamethasone. Tumors in these patients are resistant to chemotherapy, glucocorticoids, at least one proteasome inhibitor, at least one immunomodulatory drug, and CD38 antibody therapy, and their tumors continue to worsen after receiving the most recent treatment. The results of the trial showed that the objective response rate (ORR) of selinexor reached 25.4% for these highly resistant refractory MM patients. Two of the patients achieved complete remission and 29 patients achieved partial or significant partial response (VGPR). The average response time for patients was 4.4 months. At the same time, the safety and tolerability exhibited by selinexor in this trial was consistent with the results obtained in previous trials. ▲ Karyopharm Therapeutics all oral drug development pipeline (Source: Karyopharm Therapeutics official website) “The 25.4% response rate and the 4.4 month remission period are very convincing data,†said Dr. Sundar Jagannath, director of the MM program at Mount Sinai School of Medicine and professor of hematology and oncology. For oral therapy, these data suggest that selinexor may offer an exciting new treatment option for refractory patients who have tried all existing therapies." “We are very grateful to the patients and their families, as well as the researchers for their contributions to the STORM trial,†said Dr. Sharon Schacham, founder, president and chief scientific officer of Karyopharm. “We are committed to delivering this innovative oral therapy to those who cannot. In the hands of patients who benefit from existing therapies." Karyopharm plans to submit a new drug listing application (NDA) to the US FDA in the second half of this year and apply for a priority review. In early 2019, the company submitted a marketing authorization application (MAA) to the European Medicines Agency (EMA). At the same time, Karyopharm will launch a key clinical phase 3 trial called BOSTON to test the efficacy of a combination of selinexor with a proteasome inhibitor and dexamethasone in patients with MM who are resistant to one of these therapies. The patient registration process for this clinical trial is expected to be completed by the end of 2018, and the top line results are expected to be announced in 2019. We look forward to this new treatment to bring effective disease relief to MM patients as soon as possible. Reference materials: [1] Karyopharm Announces Positive Top-Line Data from Phase 2b STORM Study Evaluating Selinexorin Patients with Penta-Refractory Multiple Myeloma [2] Multiple Myeloma Flood Bag,Alternative Flood Bags,Flood Barrier Bags,Quick Dam Flood Bags Denilco Environmental technology(Suzhou)Co., Ltd. , https://www.wflood.com