Effect of molecular adsorption configuration and structure on its surface properties by dissipative quartz crystal microbalance
Shandong Longze Mechanical Equipment Co.,Ltd , https://www.pelletmachinefactory.com
The adhesion of mussel foot silk protein (Mefps) to various surfaces has been extensively studied, and 3,4-dihydroxyphenylalanine (DOPA) is considered to be the main substance for resisting wet adhesion. DOPA has both a benzene ring and a catechol group which can act on the surface of the object through a benzene ring or an o-hydroxy group, respectively, but the adhesion mechanism of the molecule on different surfaces has not been explored. Therefore, an in-depth understanding of the adhesion mechanism of DOPA and different material surfaces reveals the relationship between the structure and properties of the adsorption layer, and has important guiding significance for the design of marine antifouling and anti-corrosion functional materials.
In response to this problem, Ms. Yang Hui and Mr. Wang Jinben from the Key Laboratory of Colloid, Interface and Chemical Thermodynamics of the Institute of Chemistry, Chinese Academy of Sciences, and their team published a series of articles entitled: Adsorption and Orientation of 3,4-Dihydroxy-L -phenylalanine onto Tunable Monolayer Films" and "Construction of DOPA-SAM multilayers with corrosion resistance via controlled molecular self-assembly" are published in the Journal of Physical Chemistry C and Journal of Industrial and Engineering Chemistry , respectively. A large amount of research work has been carried out using Biolin Scientific's dissipative quartz crystal microbalance (QCM-D). This article extracts some of the contents of the article for your reference.
Measurement methods:
The research team used the quartz crystal microbalance QCM-D with the dissipative function of Swedish Bai Oulin Technology Co., Ltd. The chip was first processed, ultrasonically cleaned with SDS lotion for 10 min, rinsed with ultrapure water and dried with nitrogen, and then treated with a plasma cleaner for 10 min to remove surface organic contaminants. All thiol solutions were at a concentration of 5 mM and were dissolved in ethanol. The DOPA solution was at a concentration of 5 mM and was dissolved in NaCl/HCl (pH 5.5) buffer. The flow rate of the peristaltic pump was set to 20 μL/min.
The QCM-D technology was used to successfully assemble a series of thiol monolayers to obtain a dense and uniform molecular structure. On this basis, the DOPA solution was continuously introduced, and various DOPA/SAM multilayer films were finally constructed by various interactions between DOPA molecules and the surface and cross-linking between molecules (Fig. 1(a)). It can be seen from the thiol molecular adsorption curve in Fig. 1(b) that the adsorption process mainly includes two stages of rapid adsorption and slow rearrangement. First, the thiol molecule forms a Au-S bond with the gold surface and rapidly adsorbs to the surface. When the adsorption gradually becomes saturated, the rearrangement and configuration adjustment between the molecules causes the adsorption rate to decrease, eventually forming a smooth order. The molecular structure. Similarly, the adsorption curves of DOPA molecules on different surfaces are mainly divided into two processes of adsorption and rearrangement. Experiments have shown that the SAM-CF 3 and SAM-NH 2 surfaces promote the adsorption of DOPA molecules, while the SAM-OH and surface hinder the adsorption of DOPA molecules, probably due to the strong solvation effect of hydrophilic surfaces. 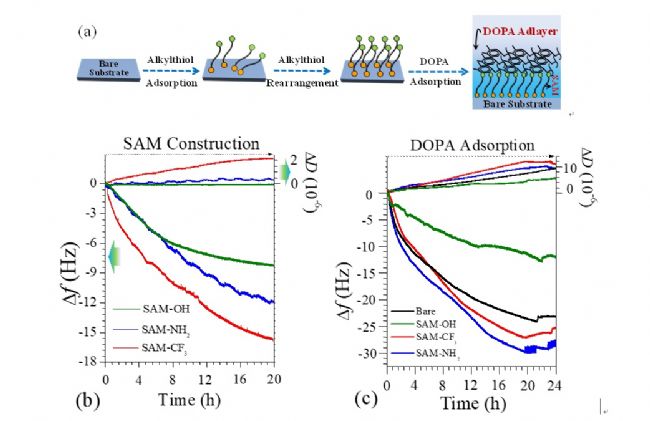
Figure 1. ( a ) Schematic diagram of the dynamic construction process of DOPA/SAM multilayer film; ( b ) The adsorption frequency and dissipation curve of thiol molecule on gold surface; ( c ) The adsorption frequency and consumption of DOPA on the surface The variation curve of the dispersion time <br> The Voigt model is used to fit the DOPA adsorption layer on different surfaces, and the adsorption quality after the coupling of water, that is, the wet mass, can be obtained, as shown in Fig. 2(a). It can be seen from the dry mass of the DOPA adsorption layer measured by ellipsometry and the water content of the adsorption layer that the DOPA/SAM-OH composite membrane couples a large amount of water to form a loose, swollen molecular layer structure. In comparison, the DOPA adsorption layer formed on the surface of SAM−NH 2 and SAM−CF 3 has a low water content, which facilitates the formation of a dense and compact molecular layer structure. The adsorption configuration and layer structure of DOPA molecules were further discussed by the Δ D −Δ f curve of the DOPA molecule on the SAM-OH surface (the lowest adsorption amount) and the SAM-NH 2 surface (the highest adsorption amount) (Fig. 2(b) ). There are two stages in the adsorption process of DOPA on both surfaces. On the SAM-OH surface, the adsorption slopes of the DOPA adsorption curve are 0.25 and 0.21, respectively, which is much higher than the adsorption slope on the surface of SAM-NH 2 , 0.19 and 0.15, further proved that DOPA forms a loose, swollen molecular structure on the surface of SAM-OH; and forms a compact, dense molecular structure on the surface of SAM-NH 2 . 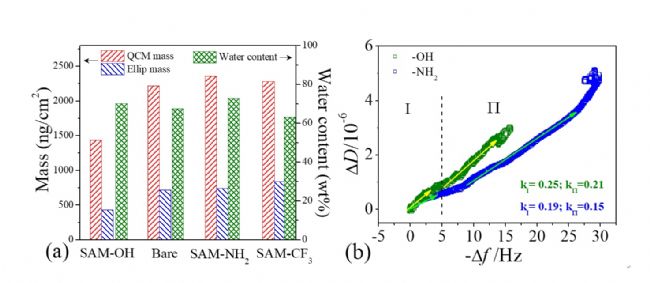
Figure 2. ( a ) Dry and wet mass and water content of different DOPA/SAM multilayer films; ( b ) D - f curve of DOPA adsorption on SAM-OH and SAM-NH 2 surfaces .
The molecular structure of DOPA molecules adsorbed on different surfaces was optimized, and the binding energy required for different molecular configurations was calculated. The results showed that DOPA was adsorbed on the surface of SAM-OH mainly in a lying molecular configuration; DOPA was in There are multiple sites of adsorption on the gold surface, so there are many adsorption configurations. On the surface of SAM−CF 3 and SAM−NH 2 , DOPA adopts a flat molecular configuration to form a dense molecular layer structure. 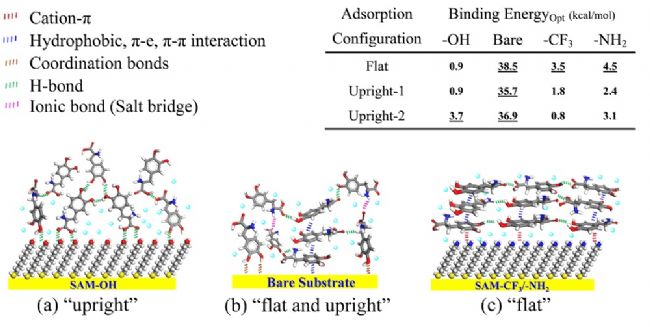
Figure 3. Schematic diagram of the binding energy and optimal molecular adsorption configuration of DOPA molecules adsorbed on different surfaces.
According to the above experiment, the strong solvation effect of hydrophilic surface such as SAM-OH can effectively block the adhesion of DOPA molecules, and preferentially form the hydrogen bond to make the DOPA molecule adopt vertical molecular configuration for adsorption and finally form. The loose adsorption layer structure; on the surface of SAM−CF 3 and SAM−NH 2 , DOPA is adsorbed by hydrophobic or cationic-π interaction, and the molecular structure of the flat layer is preferentially formed to form a dense molecular layer structure. Effectively prevent the penetration and corrosion of acidic molecules and water molecules on the surface, and greatly improve their corrosion resistance. These findings not only reveal the relationship between molecular structure and marine anti-adhesion and corrosion resistance, but also provide guidance for the design of "green" anti-adhesion and anti-corrosion functional materials.
The above results are published in the Journal of Physical Chemistry C (2017, 121, 11544-11551.) and Journal of Industrial and Engineering Chemistry (2019, 69, 179-186.). For more details, please read the original: https:/ /pubs.acs.org/doi/10.1021/acs.jpcc.7b02795; https://?via%3Dihub.