African swine fever virus nucleic acid detection program
Active Pharmaceutical Ingredients Youth Biotech CO,. Ltd. , https://www.youtherb.com
African swine fever in samples of secretions, blood, spleen, tonsils, kidneys and lymph nodes.
2. Sample pretreatment method (sample processing area)
2.1 sample pretreatment
2.1.1 Tissue sample: Each tissue is weighed about 1g from 3 different locations, and the surgical scissors are cut and mixed.
0.5g was ground in a grinder, added with 1.5mL of normal saline, and then ground. After homogenization, transfer to a 1.5mL sterile centrifuge tube, centrifuge at 8000rpm for 2min, and take the supernatant 200μL in a 1.5mL sterile centrifuge tube; The sample organization is directly used for machine extraction and is also used by the laboratory (Jiangsu Taizhou Agricultural Committee)
2.1.2 Whole blood sample: Take the supernatant (serum) of whole blood after centrifugation 200 μL in a 1.5 mL sterile centrifuge tube.
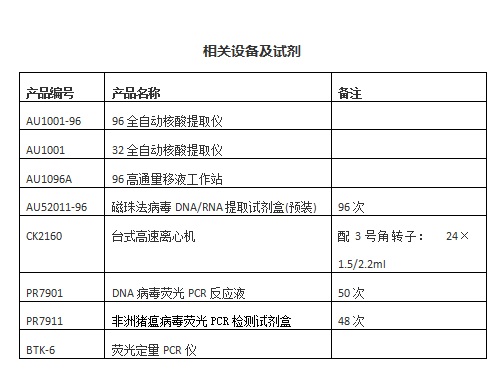
Related experimental equipment: animal tissue grinder, hand-held electric tissue grinder 
2.2 Sample storage and transportation <br> The above specimens can be stored at -20 °C in the short term and -70 °C in the long-term storage, but not more than 6 months. The specimens should be transported by blue ice and dry ice. Repeated freezing and thawing are strictly prohibited.
2. 3 DNA extraction <br> The above-mentioned processed specimens are added to a 96-deep well plate pre-packed with the extraction reagent, and the sample-added consumables are placed in a 96-automatic nucleic acid extractor to extract nucleic acids. 
The 96 automatic nucleic acid extractor is used for the extraction of viral nucleic acids. The supporting reagents can be supplied in bulk or in pre-installed form. The 96 high-throughput pipetting station can be used for the dispensing of bulk reagents.
3. Reagent preparation (reagent preparation area)
According to the total number of samples to be tested, set the number of PCR reaction tubes required to be N (N = number of samples + 1 tube negative control + 1 tube positive control; sample 7 per sample, prepare 1 more), upstream primer, downstream The primer, the fluorescent quantitative probe, and the enzyme solution are pre-mixed, and the mixed reaction solution is dispensed into the PCR reaction tube. Examples are as follows: 
4. Sample loading (sample processing area)
The extracted DNA, the positive control substance, and the negative control substance are each taken in a certain amount, and respectively added to the corresponding reaction tubes, the tube cover is covered, mixed, and briefly centrifuged. 
5. PCR amplification (nucleic acid amplification region)
5.1 placing the reaction tube to be tested in a reaction tank of a fluorescent quantitative PCR machine;
5.2 set the channel, sample information, the reaction system is set to 20μL;
Fluorescent channel selection: Reporter Dye FAM, Quencher Dye NONE, ABI series instruments Do not select ROX reference fluorescence, select None.
5.3 related equipment:
BTK-6 fluorescence PCR instrument, BTK-10 ultra-fast fluorescence PCR instrument, ABI7500, LightCycler, Bio-Rad, eppendorf and other series of real-time PCR detectors.