Pfizer Announces Closed Alzheimer's Disease and Parkinson's New Drug Development
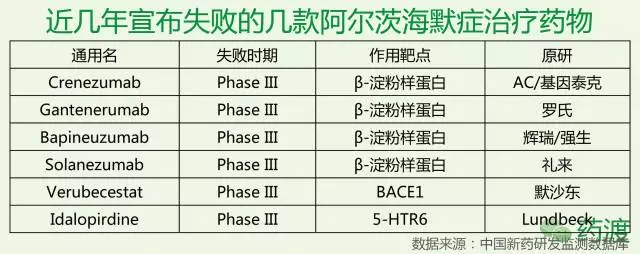
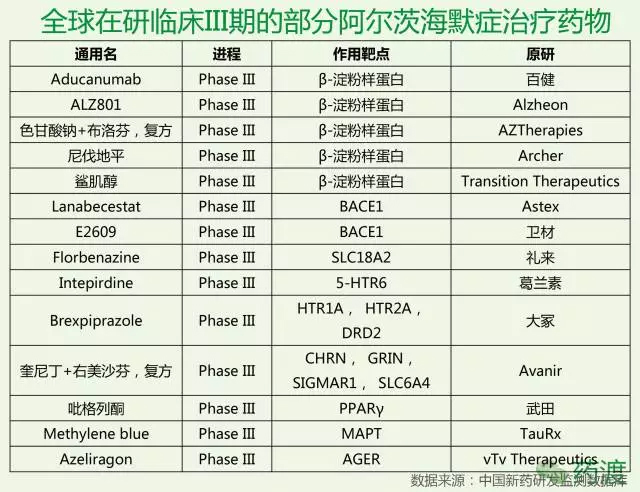
According to the latest news from The Wall Street Journal, pharmaceutical giant Pfizer said on Saturday that it would stop the discovery and research of Alzheimer's disease and Parkinson's treatment drugs, which it believes is futile and costly. To this end, about 300 people will be laid off. According to the 2015 World Alzheimer's Disease Report, there are about 46.8 million AD patients worldwide, and the number of patients is expected to double every 20 years. By 2030, it will reach 74.7 million, and in 2050 it will break through 1. 300 million people. But to the present position, since the discovery of Alzheimer's disease by humans, we have not really solved the treatment of Alzheimer's disease, and even made significant progress. Currently used in clinical medicine, mainly to relieve cognitive dysfunction and mental disorders, including cholinesterase inhibitors, excitatory amino acid receptor antagonists, Chinese medicine interventions, statins, antipsychotics, antidepressants, anti-anxiety And sedative hypnotics, etc., but most of them are not a cure for the symptoms, although it can play a certain mitigating effect, but ultimately can not control the deterioration of the disease. In October 2017, Natural Drug Discovery published an analysis of the failure of the drug research and development in Alzheimer's disease as of 2016. The article divides the 59 termination projects in the AD field (37 have sufficient public information for this analysis), 88 research projects (61 have sufficient public information), and the mechanism of action of the five listed drugs into eight categories, and then The total investment in R&D was calculated based on the number of patients recruited by various mechanisms and the clinical individual patient consumption in each phase. The authors found that the failed projects focused on the powdery protein pathway, Ta protein, neuroimmunology and neurotransmission. The drugs under study are mainly concentrated in powdered protein and Tau. 36% of the projects belong to the unclear category, while the mechanisms of pinocytosis and autophagy have almost no clinical research projects. But as we all know, so far, we have not really fully understood the pathogenesis of Alzheimer's disease. Most of the treatments currently developed are based on the above various hypotheses, so to some extent, failure is inevitable. In February 2017, Merck announced that it would stop developing the second- or third-phase clinical trial of the β-amyloid precursor protein cleavage enzyme 1 (BACE1) inhibitor verubecestat for mild to moderate Alzheimer's disease because the interim analysis showed "It is impossible to have a positive clinical benefit." However, Verubecestat's Phase 3 clinical trial for early Alzheimer's disease will continue and data is expected to be released in February 2019. In September 2017, Axovant Corporation of the United States announced that its intepirdine developed in the Phase III clinical trial for mild to moderate Alzheimer's disease did not achieve two major efficacy endpoints for cognitive ability and daily living ability. However, intepirdine will continue to conduct Phase 2 clinical trials for Lewy Body Cognition. In short, although the development of Alzheimer's disease drug is in the forefront, the blank is a huge blue ocean, and hitting the target means a huge return. In November 2016, Lilly announced that its well-recognized mild Alzheimer's disease phase III clinical drug Solanezumab did not reach the main clinical endpoint and failed. To this end, Iguchi published a Alzheimer's disease: Are we on the right path to new drugs? The article, the article is attached today for the reader's reference. Alzheimer's disease: Are we on the right path to new drugs? It is expected that the number of Alzheimer's patients worldwide will be three times higher in 2050. With the rapid increase in the number of patients, the field of drug research and development to prevent or slow down the disease is also progressing to an unprecedented height. On the 23rd last month, US pharmaceutical giant Eli Lilly announced that its Alzheimer's disease drug solanezumab failed in the phase III clinical trial. This sigh of sighs caused a lot of sensation in the industry, and the hard bones of Alzheimer's disease once again caused the industry to think. Eli Lilly Solanezumab is a monoclonal antibody based on the theory that β-amyloid plays an important role in the development of Alzheimer's disease. It slows down Alzheim by breaking down beta-amyloid plaques in the brain. The progress of the morbidity. Although the Solanezumab Phase III clinical trial ended in failure, there are still some drugs with similar mechanisms of action in an unknown long-term journey. Solanezumab is the first drug of its kind to go to the final trial phase, and researchers have been optimistic that the drug will be successful. Solanezumab's failure In 2012, early trial data showed that Solanezumab reduced the course of early Alzheimer's disease by about 34%. Last year's trial showed that the earlier the patients with Alzheimer's disease take Solanezumab, the greater the chance of success. Last month, Lilly announced that in the late-stage clinical trial EXPEDITION3, patients with mild Alzheimer's disease who received Solanezumab did not show a statistically significant slowdown in cognitive decline compared with patients treated with placebo. Solanezumab walked all the way until the test data was released, and the researchers were optimistic that the drug would be successful, which means that Lilly's new drug worth tens of billions of dollars is at your fingertips. However, Solanezumab, which has received much attention and expectation, has finally failed to break the curse of clinical trial failure. Jeremy Hughes, CEO of the British Association for Alzheimer's Disease, said: "After the good news last summer, we have high expectations for Solanezumab to become the world's first drug to slow down Alzheimer's disease, and patients with such drugs The demand is very large. It is extremely disappointing to know that Solanezumab has not brought about a significant improvement in the patient's condition." Alzheimer's Association in the United States) also pointed out in a statement that they were very disappointed with the results, but other drugs still in development, targeting β-amyloid, revealed the light of hope. However, many researchers claim that Solanezumab's failure has led to an unavoidable question: Is it really a viable path to treat Alzheimer's disease by developing new drugs with β-amyloid as a target? --amyloid and Alzheimer's disease Beta-amyloid is a "viscous" protein fragment produced by a molecule called an amyloid precursor protein. In the brain, β-amyloid can accumulate between nerve cells and gather together to form so-called plaques. This plaque is formed in some people's brains as they age, but in some areas of the brain of Alzheimer's patients, such as the hippocampus associated with learning and memory, this plaque is extremely abundant. For this reason, β-amyloid is considered to be a marker of Alzheimer's disease. However, the precise way it causes Alzheimer's disease has been a controversial topic in the scientific community. In fact, scientists still do not know whether β-amyloid plays an important role in the formation of Alzheimer's disease, or is it only a pathological condition of Alzheimer's disease. That is to say, it is not clear whether β-amyloid is the cause of Alzheimer's disease or a disease of Alzheimer's disease. Beta-amyloid is a widely accepted theory of the etiology of Alzheimer's disease. For example, in an article published in Science in 2013, the researchers used experimental mice to show how beta-amyloid disrupts the connection between nerve cells before forming plaques that cause nerve cell death. However, other studies have shown that tau may be the leading cause of Alzheimer's disease. A study published earlier this year in Science Translation Medicine found that the rich tau protein in the cerebral temporal lobe is associated with poor memory. In recent years, although Alzheimer's disease researchers have begun to increasingly use tau as a target for the development of new drugs, the focus remains more on the beta-amyloid theory. However, considering that many drugs targeting β-amyloid have repeatedly failed in the trial, the researchers' optimism about these drugs is gradually weakening. Has the β-amyloid theory died? The field of Alzheimer's disease is the hardest hit area for the failure of new drug development. According to an article published in the 2014 issue of Alzheimer's Research & Therapy, between 2000 and 2012, 244 clinically Alzheimer's drugs were only One was approved by the US FDA, and the drug development failure rate was as high as 99.6%. Most clinical trials are targeted at beta-amyloid, of which 70 out of 146 are targeted for beta-amyloid, while tau is targeted for only 13 drugs. Observing the overall statistics, it is difficult to question the feasibility of using β-amyloid-targeted drugs to treat Alzheimer's disease. Recently, the news of the failure of the Solanezumab clinical trial has not dispelled this question, but has intensified it. It is. Peter Roberts, a professor at The University of Bristol, told BBC News that he was not surprised by the failure of Solanezumab and said: "In my mind, this is a fundamental problem. There is no convincing evidence that there is a clear relationship between amyloid deposition and human cognitive deficits. What we really know is that amyloid deposition may have begun 20 years before the onset of Alzheimer's disease." Some researchers believe that the failure of solanezumab further demonstrates that beta-amyloid is a wrong target for the treatment of Alzheimer's disease. Neurologist George Perry of The University of Texas told Nature News: "The amyloid hypothesis is dead. This is a reasonable and very simple hypothesis that was proposed 25 years ago. Now it is no longer A reasonable hypothesis. A researcher from Alzheimer's disease at the Feinstein Institute for Medical Research said: "We are in vain, there is no indication that it is even a short one. In this time, the drug can make any patient better, which only indicates that the mechanism is wrong. " Maybe a different method than beta-amyloid Many scientists are still optimistic that beta-amyloid is the right target for the development of Alzheimer's drugs, but there are also some that need to re-evaluate beta-amyloid-targeted drugs. In the case of solanezumab, solanezumab binds to and eliminates β-amyloid plaques in the brain. However, Roxana O'Carare, a professor of clinical neurology from The University of Southampton, points out that this protein may need to be completely removed from the brain. Professor O'Carare explained to BBC News: "There are no lymphatic vessels in other organs in the brain. The fluids and waste in the brain are removed from the brain along very narrow channels embedded in the walls of the blood vessels. Alzheimer's risk factors appear, these channels change in composition, loss of function, and the formation of amyloid on the blood vessel wall. When a vaccine such as solanezumab works, the sticky protein plaque is eliminated. But the excess garbage and fluids are still not able to drain along the path where the lesion has occurred." Perhaps Solanezumab still has a chance to survive. Lilly said it will no longer seek listing approval for solanezumab, and the drug still needs to continue some clinical trials. An anti-amyloid treatment study (A4 study) in asymptomatic patients with Alzheimer's disease is one such trial. The trial began in 2014 and Phase III clinical trials tested the safety and efficacy of solanezumab in 1,150 patients. Although Lilly's Solanezumab fell in the last hop, some researchers and institutions believe that this is not necessarily the end of the drug. The Alzheimer's Association said in a statement: "We sincerely hope to test solanezumab and other anti-amyloid factors in the trial. These drugs act on amyloid In different ways, some drugs also show some effects on Alzheimer's disease at an early stage, and these drugs may still be effective." We can and will eventually defeat Alzheimer's disease Researchers are keen to point out that other drugs are being tested, and early trials have shown efficacy in Alzheimer's disease. The Alzheimer's Association noted that a treatment strategy under investigation is to alleviate brain inflammation or neuroinflammation, which some researchers believe may play a role in Alzheimer's disease. Earlier this year, the Alzheimer's Association announced that four new Phase I and Phase II clinical trials will each receive $1 million in funding to further explore the link between neuroinflammation and Alzheimer's disease. Dr. Maria Carrillo, Chief Scientific Officer of the Alzheimer's Association, said: "There is increasing evidence that inflammation plays an important role in the brain changes in Alzheimer's and other neurodegenerative diseases. By further understanding inflammation and The role and timing of the immune response, we will be able to further accelerate the development of new drug candidates for Alzheimer's disease." Connector Terminals,Male Crimp Circular Connector,Battery Terminal Connectors,Brass Pin Changzhou Ziying Metal Products Co., Ltd , https://www.ziyingmetal.com